- A therapy with bioactive nanoparticles acts on the blood-brain barrier and not directly on neurons.
- In mouse models, a 50-60% reduction in amyloid was achieved at the time of injection and cognitive improvement after three doses.
- The particles mimic LRP1 ligands, reactivate the natural clearance pathway, and promote the elimination of Aβ into the bloodstream.
- The approach, published in Signal Transduction and Targeted Therapy, is promising but still requires human trials.
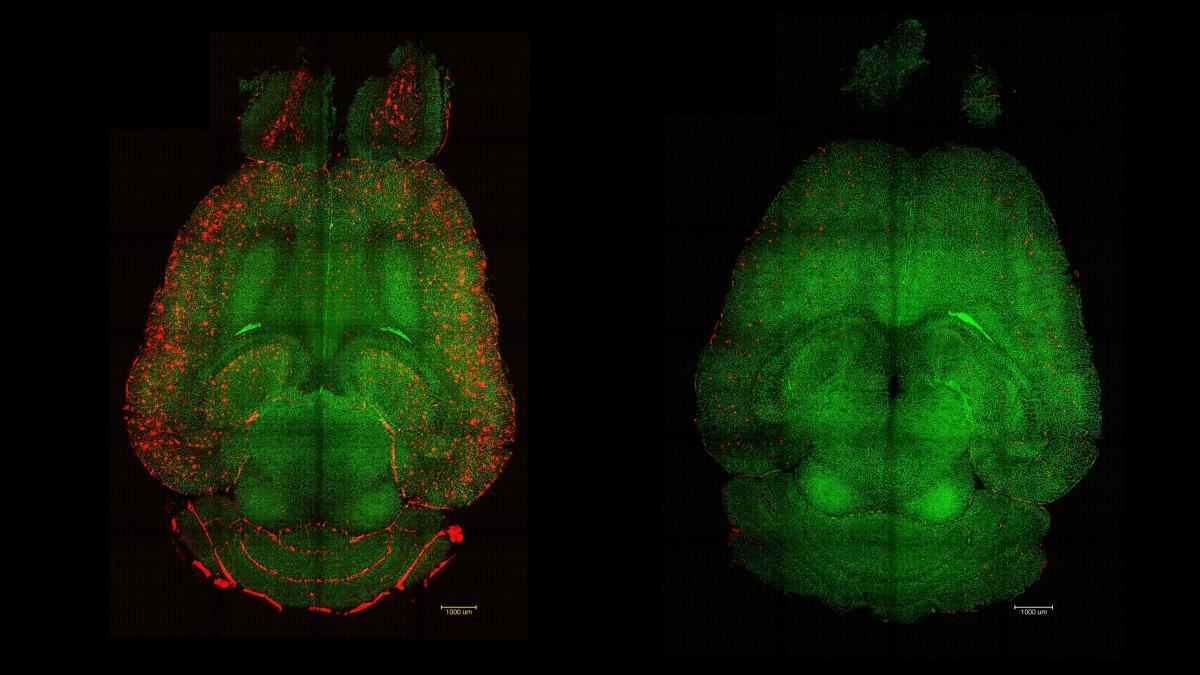
Un international team, with leadership from the Institute of Bioengineering of Catalonia (IBEC) and the West China Hospital of Sichuan University, has presented a nanotechnology strategy that reverses signs of Alzheimer's in mice by repairing the blood-brain barrier (BBB). Broadly speaking, it is about use nanoparticles that act as drugs by themselves to restore cerebral vascular function.
This shift in focus makes sense if we remember that the brain consumes about 20% of the energy in adults and even a 60% in children, supported by a dense network of capillaries where each neuron receives support. When the BBB is altered, the waste disposal system suffers and favors the accumulation of beta amyloid (Aβ), a hallmark of the pathologyIt is estimated that the human brain contains around one billion capillaries, hence the importance of vascular health.
What does this nanotechnology strategy propose?

Unlike classical nanomedicine, which uses nanoparticles as mere vehicles, this approach employs supramolecular drugs which are bioactive and do not require transporting another principle. The target is not the neuron, but the BBB as a therapeutic target.
Under normal conditions, The LRP1 receptor recognizes Aβ and transfers it across the barrier into the bloodstreamThe system, however, is delicate: If the binding is excessive or insufficient, transport is unbalanced and Aβ accumulates. The designed nanoparticles mimic LRP1 ligands to regain that balance.
With this intervention, the exit route of problematic proteins from the parenchyma into the blood, promoting Aβ clearance and normalizing barrier function. In short, it reactivates the natural cleansing route of the brain.
Animal model testing and results
The evaluation was carried out on mice genetically modified to produce large amounts of Aβ and develop cognitive impairment. Three injections of these particles were enough to observe measurable changes in biomarkers and behavior..
According to the authors, just one hour after administration A 50-60% decrease in Aβ in the brain has already been recordedThe rapidity of the effect suggests an immediate reactivation of the transport mechanism across the barrier.
Beyond the immediate impact, lasting effects are described. In one experiment, a 12-month-old mouse was re-evaluated at 18 months and showed performance similar to that of a healthy animal, indicating sustained functional recovery after treatment.
The team interprets that there is a chain effect: by restoring vascular function, The clearance of Aβ and other harmful molecules resumes, and the system regains its balance.. In the words of scientific leadership, the particles act like a drug that reactivates the elimination pathway to normal levels.
External specialists describe the discovery as promising, although they point out that the results have been obtained in murine models and that translation to patients requires caution. The community emphasizes the need to verify safety and efficacy in humans with rigorous studies.
Molecular engineering behind nanoparticles
These nanoparticles are conceived with an approach of bottom-up molecular engineering, combining a controlled size with a defined number of ligands on its surface to interact with receptors in a specific way.
By modulating the receptor traffic in the membrane, The particles fine-tune the process of Aβ translocation across the BBBThis degree of precision opens up avenues for regulate receptor functions which until now were difficult to manipulate therapeutically.
Thus, not only is the effective elimination of Aβ promoted, but It helps rebalance vascular dynamics that support healthy brain function.. This is a key difference from approaches that are limited to deliver medications.
Who's participating and what's next?
The consortium brings together the IBEC, West China Hospital and Xiamen West China Hospital of Sichuan University, the University College London, the University of Barcelona, ICREA and the Chinese Academy of Medical Sciences, among others. The findings have been published in the journal Signal Transduction and Targeted Therapy.
In view of the translation, the logical itinerary goes through independent validations, Toxicological studies, dose analysis and, if appropriate, phase I/II human trialsSafety and reproducibility will be key to moving forward.
Beyond Alzheimer's, this work focuses on the cerebrovascular health as a key element of dementia, opening a therapeutic field that complements classical neuron-centered approaches.
The data set suggests that intervening on the blood-brain barrier with bioactive nanoparticles can rapidly reduce amyloid load, restore vascular function, and improve cognitive outcomes in mice; a promising avenue that, with due caution, should be confirmed in Clinical studies well designed.
I am a technology enthusiast who has turned his "geek" interests into a profession. I have spent more than 10 years of my life using cutting-edge technology and tinkering with all kinds of programs out of pure curiosity. Now I have specialized in computer technology and video games. This is because for more than 5 years I have been writing for various websites on technology and video games, creating articles that seek to give you the information you need in a language that is understandable to everyone.
If you have any questions, my knowledge ranges from everything related to the Windows operating system as well as Android for mobile phones. And my commitment is to you, I am always willing to spend a few minutes and help you resolve any questions you may have in this internet world.